Abstract
Introduction: Daratumumab (DARA) is a human, CD38-targeted, IgGκ monoclonal antibody. In the CASTOR study, D-Vd reduced the risk of disease progression or death by 68% and induced higher rates of deeper responses vs Vd in relapsed/refractory (RR) MM pts (Spencer, A. ASH 2017. Abs. 3145). Overall, in phase 3 studies in RRMM and newly diagnosed MM, DARA-based regimens reduced disease progression or death risk by ≥50%, doubled complete response (CR) rates, and tripled minimal residual disease (MRD)-negative rates. While progression-free survival (PFS) benefits of D-Vd vs Vd were observed regardless of the number of prior lines (PLs) of therapy, the benefit was most pronounced in pts receiving 1 PL of therapy. Here, we examine updated (2 y after interim analysis) efficacy and safety of D-Vd vs Vd in CASTOR, with a primary focus on pts with 1 PL of therapy.
Method: Pts in CASTOR were randomized to receive 8 cycles (21 d/cycle) of V (1.3 mg/m2, SC) on Days 1, 4, 8, and 11 and dexamethasone (20 mg, PO or IV) on Days 1, 2, 4, 5, 8, 9, 11, and 12 with or without DARA (16 mg/kg, IV) given weekly for Cycles 1-3, Q3W for Cycles 4-8, and Q4W thereafter. Cytogenetic risk was evaluated centrally by next generation sequencing; high risk was defined as having t(4;14), t(14;16), and/or del17p abnormalities. MRD was assessed at the time of suspected CR and at 6 and 12 mo following the first treatment dose, and an additional MRD evaluation was required every 12 mo post-CR. MRD was evaluated using clonoSEQ® V2.0 (Adaptive Biotechnologies, Seattle, WA). Sustained MRD negativity was defined as maintenance of MRD negativity at 10-5 for ≥6 or ≥12 mo.
Results: At the clinical cutoff date of January 11, 2018, 498 pts were included in the intent-to-treat (ITT) population (D-Vd, n = 251; Vd, n = 247). Pts received a median of 2 (1-10) PLs of therapy including 235 pts that received 1 PL (D-Vd, n = 122; Vd, n = 113). In the ITT population, 61% received prior ASCT, 66% V, 42% lenalidomide (R), and 32% were refractory to their last PL of therapy. Among 1 PL pts, 60% received prior ASCT, 51% V, 20% R, and 18% were refractory to their last PL of therapy.
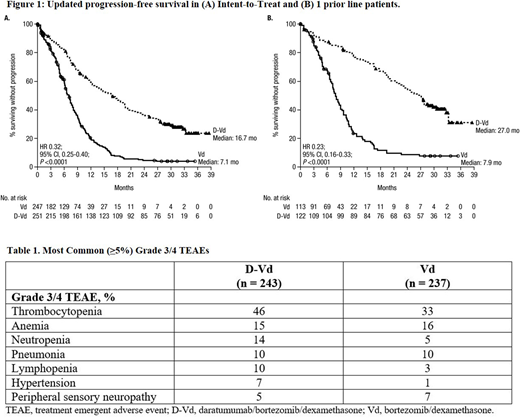
After a median follow-up of 31.3 mo, PFS was significantly prolonged with D-Vd compared with Vd in the ITT population (median: 16.7 vs 7.1 mo; HR, 0.32; 95% CI, 0.25-0.40, P <0.0001; Figure 1A). PFS benefit for D-Vd vs Vd was maintained in pts with high (median: 11.2 vs 7.2 mo; HR, 0.45; 95% CI, 0.25-0.80, P <0.01) and standard cytogenetic risk (median: 18.0 vs 7.0 mo; HR, 0.27; 95% CI, 0.19-0.38, P <0.0001). At the time of analysis, 88 deaths in D-Vd and 101 deaths in Vd were observed, and follow-up is ongoing. The overall response rate (ORR; 85% vs 63%), ≥very good partial response (VGPR) rate (63% vs 29%) and ≥CR rate (30% vs 10%) were all significantly higher (all P <0.0001) with D-Vd vs Vd. Deeper responses with D-Vd translated to higher MRD-negative rates at 10-5 for the ITT population (14% vs 2%; P <0.0001) and in both cytogenetic risk groups (high risk: 18% vs 0%; P <0.01; standard risk: 15% vs 2%; P <0.0001). Among ITT pts, sustained MRD negativity was maintained in 22 (9%) D-Vd vs 3 (1%) Vd pts for ≥6 mo, and 8 (3%) D-Vd vs 0 Vd pts for ≥12 mo.
Among 1 PL pts, median PFS was 27.0 vs 7.9 mo (HR, 0.23; 95% CI, 0.16-0.33, P <0.0001; Figure 1B) for D-Vd vs Vd. PFS benefit for D-Vd vs Vd was maintained among 1 PL pts previously exposed to V (median: 20.4 vs 8.0 mo; HR, 0.22; 95% CI, 0.13-0.37; P <0.0001) or R (median: 21.2 vs 7.0 mo; HR, 0.30; 95% CI, 0.11-0.82; P = 0.01). For 1 PL pts, 28 vs 41 deaths were observed for D-Vd vs Vd. ORR (92% vs 74%), ≥VGPR (77% vs 42%), and ≥CR (43% vs 15%) rates were significantly higher (all P <0.001) with D-Vd vs Vd. MRD-negative rates at 10-5 were also significantly higher for D-Vd vs Vd (20% vs 3%; P <0.0001), and sustained MRD negativity was observed in 8 (7%) vs 1 (0.9%) pts at ≥6 mo cutoff and 7 (6%) vs 0 pts at ≥12 mo cutoff, respectively.
The most common (≥5%) grade 3/4 treatment-emergent adverse events (TEAEs) are in Table 1. Discontinuation rates due to TEAEs were similar for D-Vd vs Vd (10% vs 9%). Second primary malignancy rates were 5% vs 2%, respectively.
Updated data will be presented.
Conclusions: In this 2-y update, D-Vd maintains significant PFS and ORR benefits in RRMM pts, with greater benefit in 1 PL pts. Addition of DARA to Vd allows for sustained MRD negativity. The safety profile of D-Vd remains consistent after 2 y. The data suggest that administration of D-Vd to RRMM pts after first relapse may provide the greatest clinical benefit.
Mateos:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Sonneveld:Amgen: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Hungria:Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Takeda: Honoraria. Nooka:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Spectrum Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptive technologies: Consultancy, Membership on an entity's Board of Directors or advisory committees. Estell:Janssen: Membership on an entity's Board of Directors or advisory committees. Corradini:Celgene: Honoraria, Other: Advisory Board & Lecturer; Janssen: Honoraria, Other: Lecturer; Roche: Honoraria, Other: Advisory Board & Lecturer; Amgen: Honoraria, Other: Advisory Board & Lecturer; Novartis: Honoraria, Other: Advisory Board & Lecturer; Sandoz: Other: Advisory Board; Abbvie: Honoraria, Other: Advisory Board & Lecturer; Sanofi: Honoraria, Other: Advisory Board & Lecturer; Takeda: Honoraria, Other: Advisory Board & Lecturer; Gilead: Honoraria, Other: Advisory Board & Lecturer. Weisel:Amgen, Celgene, Janssen, and Sanofi: Research Funding; Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen, BMS, Celgene, Janssen, and Takeda: Honoraria. Chiu:Janssen Research & Development, LLC: Employment. Schecter:Janssen Research & Development, LLC: Employment. Amin:Janssen Research & Development, LLC: Employment. Qin:Janssen Research & Development, LLC: Employment. Qi:Janssen: Employment. Spencer:Celgene: Honoraria, Research Funding, Speakers Bureau; Janssen-Cilag: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding; BMS: Research Funding; Takeda: Honoraria, Research Funding, Speakers Bureau; STA: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.